The new EU In-vitro Diagnostic Regulation (Regulation EU 2017/546, IVDR) is a European Union regulation that governs the requirements for in-vitro diagnostic (IVD) devices. It entered into force on May 26, 2017, replacing the previous EU Directive on in-vitro diagnostic medical devices (Directive 98/79/EC, IVDD). Manufacturers had a transition period of five years, until May 26, 2022, to implement the revised IVDR requirements for CE-marked in-vitro diagnostic devices. The regulation is legally enforceable in all member states of the European Union and the European Free Trade Association (EFTA).
IVDR Requirements
The IVDR places more stringent requirements on manufacturers to ensure the safety, performance, and usefulness (known as performance evaluation) of devices. Among other things, the regulation requires rigorous review of technical documentation and clinical evidence to ensure that IVD products meet essential safety and performance requirements. Manufacturers must also demonstrate a quality management system and conduct ongoing risk assessments, as well as demonstrate the lifetime safety of their products.
The IVDR also brings changes for Notified Bodies, which are responsible for approving IVD products. Notified Bodies must now meet higher requirements and will be monitored by national authorities on a regular basis. The monitoring of IVD products will be more stringent as well. For example, from now on, a Notified Body must be involved for products in risk class B (medium risk level) and higher (risk classes C and D). This automatically increases the number of products that need to be monitored. Thus, the IVDR represents a major challenge for IVD product manufacturers, but also offers benefits for patients’ safety and the healthcare system. The stricter requirements for IVD products will help ensure that only high quality and safe products will be placed on the market. This, in turn, will help ensure that patients receive a more accurate diagnosis and that treatment can be adjusted accordingly.
IVDR Classification
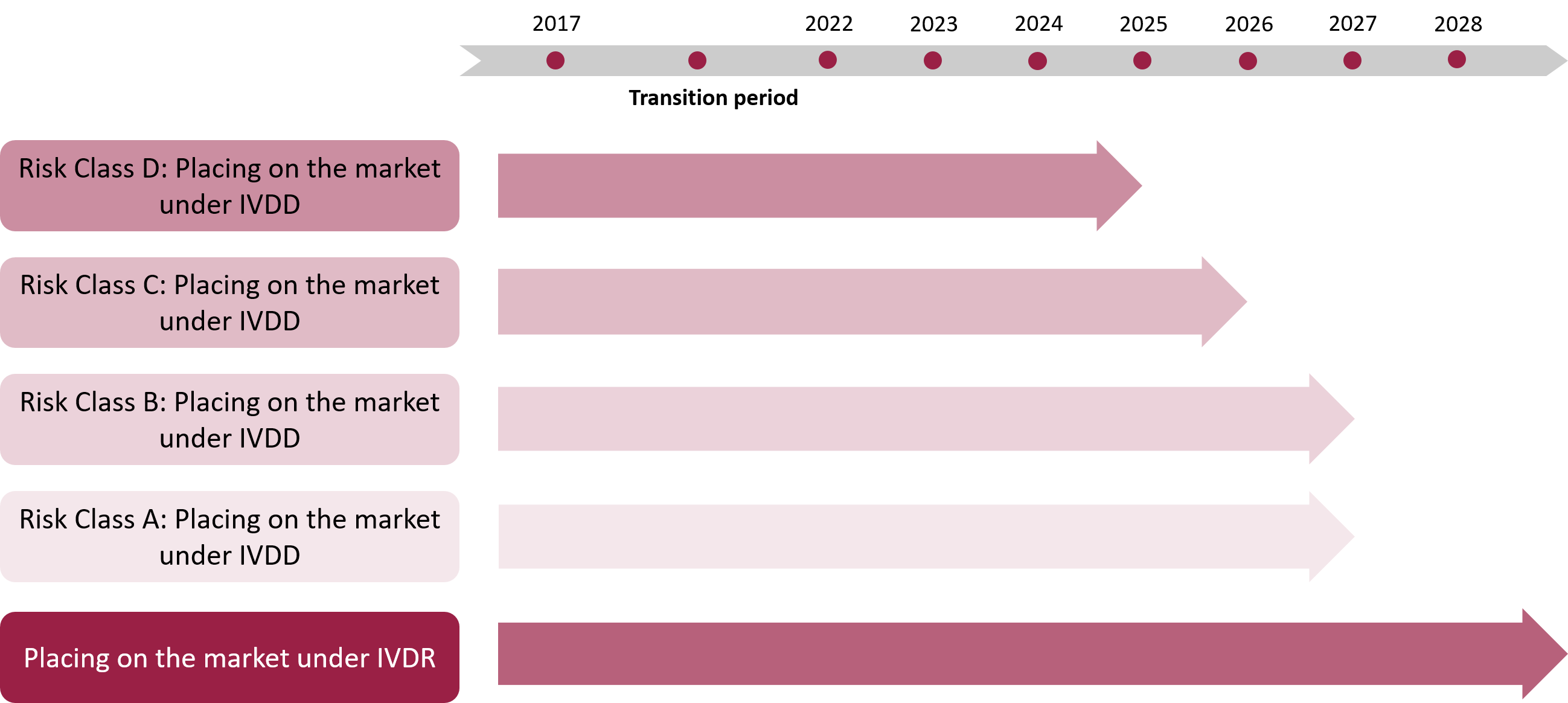
All in-vitro diagnostic devices are classified into classes A, B, C or D according to IVDR, instead of two lists (A and B) so far. The classification process is mainly based on the intended purpose of the devices and the resulting expected risks.
It is now very important to know the class of the device at early stages of development, because under the new regulations the applicable conformity assessment procedure changes depending on the class.
You can find further information on the correct classification of medical devices and in-vitro diagnostic medical devices here.
