Risk Classification of Medical Devices
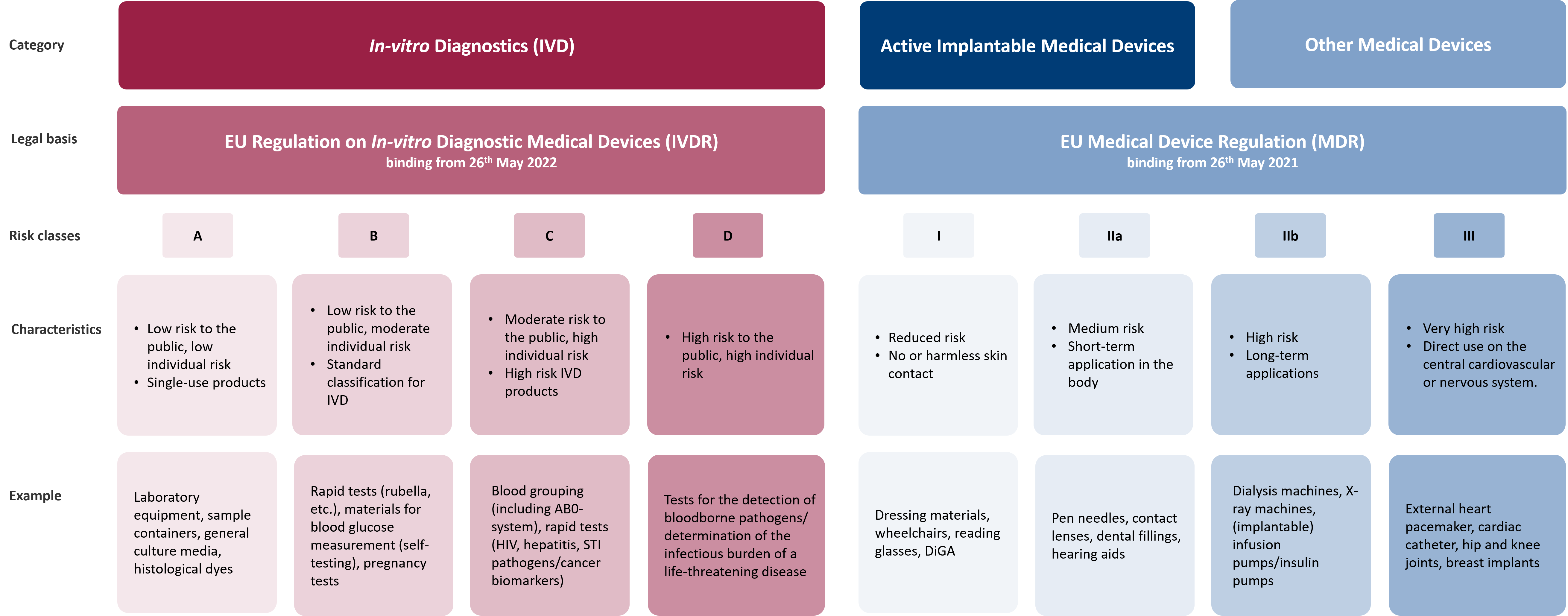
Medical devices are classified into new different risk classes in the MDR and IVDR. The aim of this risk classification is to define the risk of each product for the user and third parties. Here, a distinction is made between in-vitro diagnostic devices and other medical devices, such as actively implantable products or DiGA. Both product groups are divided into different risk classes and are subject to different requirements for placing on the market and post-market surveillance, depending on the legal background.
Determining the risk class of your medical device is one of the first steps you must consider when designing and developing a new product for the EU market. Depending on the risk class of your product, you will need to provide different evidence to obtain CE label and successfully launch your product on the market.
Classification of Medical Devices According to the MDR (EU) 2017/745
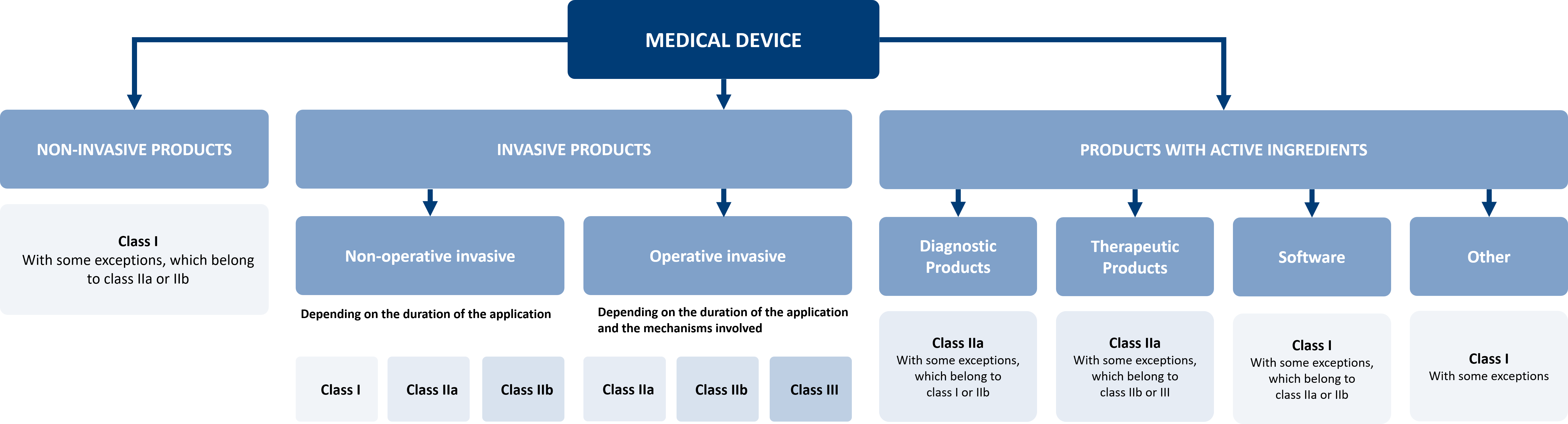
Active implantable medical devices, DiGA and other medical devices are divided into four different risk classes by means of the MDR: Class I, Class IIa, Class IIb and Class III, with Class III having the highest risk.
The MDD already contained a risk classification of medical devices. This has been updated under the new MDR and amended in several parts. Therefore, it is also necessary for manufacturers of an already certified medical device to check the current risk class of your product, to adjust it if necessary and to submit any missing clinical data as soon as possible.
In the new MDR, you will find the rules for risk classification in Annex 8. However, determining the correct risk class can be difficult due to the numerous and complex details. Therefore, we have developed a classification guide for you, which supports you in determining the risk class of your product.
Classification of In-vitro Diagnostic Medical Devices According to the IVDR (EU) 2017/746
Contrary to the previous categorisation into Lists A and B, the IVDR now mandates the classification of in-vitro diagnostics into Classes A, B, C, or D. This classification process primarily relies on the intended purpose of the products and the associated risks.
Below, we provide a comprehensive list of all classification rules according to the IVDR. If multiple provisions are applicable to the same product, it is advisable to err on the side of caution and select the higher risk class.

Do you need further assistance?
We are happy to help you classify your medical devices correctly! Contact us today at info@mic-mainz.de or +49 6131 17-9646 and get comprehensive advice from our experts!
